Abstract
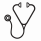
Introduction: GZL is described as sharing features with both cHL and DLBCL. It was first described in 2005 and included in the 2008 and 2016 WHO classifications. However, there remains complexity in establishing diagnosis, determining optimum therapy and delineating prognosis.
Methods: To further define diagnostic features and to establish clinical correlations, 73 GZL cases diagnosed at 15 North American academic centers (62 cases from Evens et al. Am J Hematol 2015 and 11 subsequent cases) were obtained (ie, full sections/slides/tumor blocks) and triaged centrally. Criteria for GZL diagnosis followed 2016 WHO classification. All diagnostic samples were evaluated with a minimum immunohistochemistry (IHC) panel: CD20, CD79a, PAX5, OCT2, BCL6, MUM1, CD30, CD15, CD3 and EBV in situ hybridization. Beyond IHC, diagnostic criteria included tumor cell density and morphology, necrosis and microenvironment. Cases were evaluated by 5 expert hematopathologists and consensus diagnosis was reached at a multi-headed scope; 5 cases were rejected for insufficient material. 3-year survival rates were estimated by Kaplan-Meier with differences assessed by log rank test. Univariate associations of clinical and lab factors with survival were derived using Cox proportional hazards model with variables P ≤0.10 entered stepwise into a multivariate (MVA) Cox hazards model.
Results: Of the 68 original cases diagnosed as GZL, only 26 (38%) were confirmed as GZL on central review. Both architectural and cytological features were important for diagnosis of GZL where tumor cell content was high, and while occasional cells resembled HRS cells, the spectrum in cytological appearances was very broad, including some cells with centroblastic or immunoblastic cytology. Marked nuclear pleomorphism was a characteristic feature. A smaller subset of GZL cases had more monomorphic cytology, resembling primary mediastinal B-cell lymphoma (PMBL), but lacked classic B-cell phenotype. IHC of GZL cases showed universal B-cell derivation (positive for ≥1 B-cell marker: CD20, CD79a and/or PAX5), frequent CD30 expression, and rare EBV positivity (ie, CD20+ 83%; PAX5+ 100%; BCL6+ 20%; MUM1+ 100%; CD30+ 92%; EBV+ 4%). 42 cases were reclassified as: nodular sclerosis (NS) cHL, n=27 (including n=10 NS grade 2 (G2)); nodular lymphocyte predominant HL, n=4; DLBCL, n=4; EBV+ DLBCL, n=3; PMBL n=2; lymphocyte-rich cHL and BCL-NOS, n=1 each. Of confirmed GZL cases, clinical features included: median age 37 years (19-72); M:F 1.9:1; ECOG PS 0-1 in 88%; anemia 58%, increased LDH 35%; hypoalbuminemia 57%. None had >1 extranodal site or bone marrow involvement; 69% had primary mediastinal disease; 65% had early-stage disease with 33% bulk. Only a minority of pts had high IPI or IPS (19% and 7%, respectively). GZL confirmed vs re-classified cases more often had mediastinal disease (69% vs 41%, P= 0.04) and less often had >1 extranodal site (0 vs 25%, P= 0.02). With 44-month median follow-up, 3-year progression-free survival (PFS) and overall survival for GZL patients (pts) were 39% and 95%, respectively, vs 58% and 85%, respectively, for re-classified cases (P= 0.19 and P= 0.15, respectively) (Fig. 1A). Interestingly, NS-G2 re-classified pts had similar 3-year PFS (ie, 43%) as GZL consensus confirmed cases. For prognostication of GZL consensus confirmed pts, moderate/strong expression of CD30 and hypoalbuminemia were negative factors (Fig. 1B/C) and frontline cyclophosphamide, doxorubicin, vincristine, prednisone +/- rituximab (R-CHOP) vs other therapy (primarily ABVD) was associated with improved PFS (Fig. 1D). On Cox regression MVA (including controlling for IPI), moderate/strong CD30 expression was borderline (PFS hazard ratio (HR) 7.08, P=0.06) and hypoalbuminemia remained significant (PFS HR 5.54, P= 0.03).
Conclusions: Altogether, accurate diagnosis of GZL remains challenging and improved therapeutic strategies are needed. High tumor cell content is very helpful when differentiating between GZL and cHL, the most common alternative diagnosis. Additionally, CD30 expression was common, while EBV positivity was rare. The small number of pts and relative heterogeneity of therapy limit definitive delineation of optimal therapy for GZL; however, our results suggest that DLBCL based therapy was most effective, including R-CHOP. Finally, we identified prognostic factors that identified GZL pts with divergent clinical outcomes.
Evens:Amgen: Consultancy; Pharmacyclics: Consultancy; Seattle Genetics: Consultancy; Novartis: Consultancy; • Spectrum Pharmaceuticals: Consultancy; Merck: Consultancy; Kite Pharma: Consultancy; Millennium: Consultancy; Celgene: Consultancy; AbbVie: Consultancy; Affimed: Consultancy. Feldman:AbbVie: Speakers Bureau; Celgene: Speakers Bureau; Pharmacyclics: Speakers Bureau; Janssen: Speakers Bureau; Bristol-Myers Squibb: Consultancy; Kite Pharma: Speakers Bureau; Seattle Genetics: Honoraria, Research Funding, Speakers Bureau. Lossos:Affimed: Research Funding. Abramson:Genentech: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy. Press:Roche: Honoraria, Research Funding; BMS: Honoraria; Bayer: Consultancy. Fenske:Sanofi: Consultancy; Celgene: Consultancy; Pharmacyclics: Consultancy. Friedberg:Bayer HealthCare Pharmaceuticals.: Other: Data and Safety Monitoring Board: Bayer HealthCare Pharmaceuticals.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal